Abstract
Background: The 2016 revision of the World Health Organization classification of diffuse large B cell lymphoma (DLBCL) takes into consideration the cell of origin (COO), germinal center B-cell (GCB) vs non germinal center/activated B-cell type (non-GCB/ABC), since its determination by gene expression profiling predicts prognosis when treated with standard therapy. Several new COO directed therapies are being investigated, mostly using immunohistochemically (IHC) staining as surrogates. However, IHC is subject to many uncontrolled variables. In this report we evaluated the impact of choice of therapy on the outcome of ABC subtype in our institution determined by IHC using Hans algorithm.
Methods: We reviewed the pathology reports of patients with DLBCL diagnosed between 2009- 2016. Cases were sub classified using CD10, bcl-6, and MUM1 expression by IHC per Hans algorithm. We excluded post-transplant lymphoproliferative disease, and primary central nervous system lymphomas. Clinical details including age, sex, International Prognostic Index (IPI) stage at diagnosis, treatment, progression free survival (PFS) and overall survival (OS) were captured for all patients. Actuarial survival analysis was performed according to the Kaplan and Meier method.
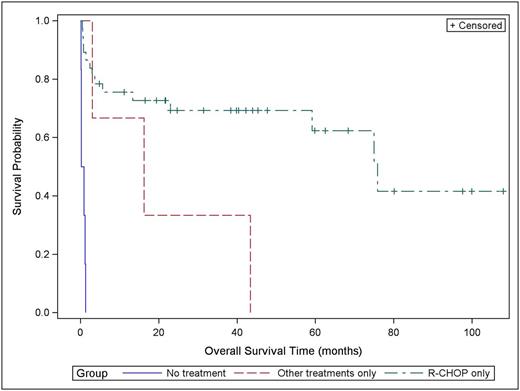
Results: We identified 285 patients with DLBCL. Per Hans algorithm, 140 (49.1%) were of GCB, 94 (32.9%) non-GCB/ABC and 51 (18%) indeterminate. For the non-GCB/ABC group, median age at diagnosis was 67 (range, 21 to 101 y) with 67% of the patients older than 60 years. 77% of the patients were Caucasians. The gender distribution was equal. ECOG performance status was < 2 in 69%. 58% of the patients had Ann Arbor stage III and IV disease. Lactate dehydrogenase (LDH) was high in 64% of the patients. There was more than one site of extranodal disease in 42% of the patients. 47% of the patients had IPI score of 0-2; while 53% had score of 3-5. Double expressers who were positive for MYC and BCL-2 identified in 19% of the patients. Frontline therapy was R-CHOP (Rituximab plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone) in 78% of the patients. Patients who received other treatments were 19 (20%), 2 of those received EPOCH ( Etoposide, Prednisone, Vincristine, Cyclophosphamide, and Doxorubicin) and the rest received either BR (Bendamustine plus Rituximab) or Rituximab or radiation by itself due to poor functional status or comorbidities. 21% of the patients needed multiple lines of treatment. There were 8 patients (13%) who had autologous stem cell transplant (ASCT). Complete response was achieved in 76% of all patients. A median survival time for all patients was 59.2 month (95% confidence interval (CI): 13.4-N/A), the 2 year survival probability 56.6% with standard error of 6.9. Median OS for patients who received R-CHOP alone was 75.8 month (95% CI: 59.2-N/A) and the 2-year survival probability for that group was 68.6% with standard error of 7.9. For patients who received treatments other than R-CHOP, the median OS for that group is 16.2 month (95% CI: 3.0-43.4) (Figure 1). Patients who did not receive any treatments had median OS of 0.56 month (95% CI: 0.13-1.32) with zero survival probability. Median duration of remission for patients who received R-CHOP was 2 years (95% CI: 1.0-5.0) and was 1.25 years (95% CI: 0.3-6.0) for patients who received other treatments. Double expressers available for clinical follow up were 9 patients, and all received R-CHOP as frontline therapy and their median OS was 5.6 month (95% CI: 0.5-N/A) with 2 year probability survival of 41.2% with standard error of 16.9. Of the patients who received ASCT, 6 patients stayed in complete remission after transplant and 1 was in partial remission and 1 progressed after transplant. Our results are comparable to previously reported studies (Molina, TJ, et al. 2014).
Conclusions: In our institution, non-GCB/ABC DLBCL patients identified by IHC who received R-CHOP had better outcome than other treatments. However, the median duration of first remission in that group was only 2 years. Clinical trials adding newer agents are warranted in this group. Yet, COO was indeterminate by IHC in 18% of the patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal